Brain Cancer in Kids: Tailoring Treatment Based on Mutations
I’ll admit it, I was sucked in.
“Sharon was given a few months to live if her cancer wasn’t treated,” somberly intones the voiceover. Then the non-descript older woman sitting tall on a plain chair tells her story – she had non-small cell lung cancer, but, thanks to Keytruda, she’s alive a year later. The camera pans to a young relative off to the side, her eyes brimming.
When a new story extolling Keytruda appeared, starring Donna, I began to fret that something dire had befallen Sharon. It was then that I noticed, at the bottom of Donna’s story, the words “Donna is a real patient.”
Was Sharon an avatar? I went back to her ad and noticed, for the first time, the scroll at the bottom of the screen: “Actor portrayal of a real patient from the clinical trial.”
Oops.
Both patients on Keytruda, however, are better off than the older gent pointing up to a skyscraper in another ad on TV, beautiful wife by his side. He also had non-small cell lung cancer, but took the drug Opdivo, which gave him “a chance to live longer.” Bristol-Myers Squibb took some flak for initially omitting the fact that “live longer” meant about 3 months, info now included in very small print.
TREATMENTS TARGET MUTATIONS IN CELLS, NOT PEOPLE
Keytruda and Opdivo awaken or rev up the immune response against tumor cells. They’re different monoclonal antibodies that attack a protein called PD-L1 that normally shuts off that response. (Specifically, PD-L1 proteins on tumor cells bind PD-1 proteins on immune system cells, like snap beads, and that lifts the normal dampening of T cell action.) But Merck’s Keytruda has fared better – leading to longer survival, like in Donna and Sharon – because the clinical trial was restricted to patients in whom more than 50% of their cancer cells had lots of PD-L1s. Opdivo’s clinical trial participants were not as well scrutinized for the genetic characteristics of their tumor cells.
The protein topography of cancer cells, determined by their genes, is so important in selecting treatment that it’s now considered along with or even before body part. The idea isn’t entirely new. I wrote “Mutation and Location Important in Cancer Treatment” for Lancet Oncology two years ago, and the genetic glitches in cancer cells have been studied for decades.
Cancer isn’t a genetic disease, like cystic fibrosis or hemophilia with a mutation in every cell, but cancer cells proceed through a choreography of diverse mutations. Oncogenes turn on, tumor suppressor genes turn off, and bits of chromosomes are jettisoned while others repeat repeat repeat.
Therapies targeted to the genetic changes in cancer cells can now be first-line treatment. That’s new. So now some patients don’t waste months and years suffering through brutal and futile standard chemo, as my mother and father-in-law did. Metastatic melanoma with cells mutant for the V600E variant of the gene BRAF is a good example. The targeted drugs Zelboraf (vemurafenib) and Tafinlar (dabrafenib) are now initial treatments for some patients.

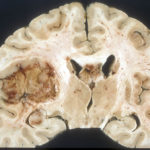
PEDIATRIC GLIOMAS = 10 CONDITIONS, NOT 1
The first step in taking a genetic approach to classifying cancer has come to pediatric brain tumors (gliomas), for which median overall survival is 9 to 15 months from diagnosis. One reason for that dire statistic is a past focus on testing drugs that help adults on kids, whose tumors tend to be in different parts of the brain from adults. Brain tumor cells from an adult and a child that appear the same histologically often in fact harbor different mutations.
An article just published in Cancer Cell identified stark genetic differences among more than 1,000 high grade brain tumors in kids and young adults, reclassifying the condition into ten distinct subtypes. And that means good news for some families. I contacted the principal investigator.
“High grade glioma, at least in children, is in fact a remarkably diverse group of tumors. Many are not really biologically or clinically high grade, and should not really be considered as such. Some have single gene fusion events, possibly targetable, while others have the highest mutation burden in human cancer, but may be excellent candidates for immune therapies. A simple microscope-based diagnosis is absolutely not sufficient to determine which subtype any child may have,” Chris Jones, PhD, leader of the glioma team at The Institute of Cancer Research in the UK , told me.
The World Health Organization has led the way in reclassifying childhood brain tumors. In 2016 they deemed “diffuse midline glioma with mutation in a histone gene (H3K27)” a distinct clinical entity. Adding a stain that binds to the histone proteins in cancer cells on a microscope slide reveals the subtype, which otherwise resembles other types of glioma, the cells large and with several nuclei. H3K27 cancers arise in the pons, affect younger kids, and patients survive slightly longer than patients with other types of brain cancer.
Histones are the spool-like proteins around which DNA wraps, and they control which genes are accessed or silenced. So a mutation in a histone protein can trigger a suite of gene expression changes – lifting the control over cell division that is cancer.
“REMARKABLE BIOLOGICAL DIVERSITY” AMONG GLIOMA CELLS
The new research took a deep dive into the normal functions of the genes that go haywire in cancer, an approach termed “integrated pathway enrichment analysis.” And many activities go astray, at several levels:
• Chromosomes replicating and then pair parting as a cell divides
• DNA repair
• Patterns of histones on DNA
• The immune response
• Cell movement
• Development of the brain and communication among its cells
• Specialization of neurons and the types of glia, covered in last week’s post
• Shuttling of stuff into and out of cell nuclei
• Cells sending and receiving signals
The analysis revealed aspects of tumor biology that suggested that certain drugs would work, and certain others wouldn’t. And the researchers were surprised at how strictly mutations segregated. Three examples of possible clinical impact for the childhood gliomas:
• Tumor cells with mutations in the genes BRAF or NF1, or several specific fusions of chromosome parts, have the best overall survival, median 63 months. This is because the cells readily communicate normally and tend to maintain their boundaries.
• Cells with extra chromosome 2 material or extra copies of the genes EGFR, CDK6, or MYCN have the worst median survival, 18 months.
• Gliomas arising in young adults with losses to chromosomes 1 and 2, a gain in chromosome 17, and extra copies of the genes PDGFRA and MET have a median survival of 18 months.
But predicting survival isn’t all that the genetic classification of cancer cells can do.
Further bioinformatics analysis can match mutations and their associated perturbed functions with the ways that new targeted drugs work, enabling doctors to more often pick the best treatment right after diagnosis and surgery. And the new DNA-based classification will rationalize the design of clinical trials to evaluate new treatments, so that future cancer patients can see many more tomorrows – like Donna and Sharon.



[…] Source: Brain Cancer in Kids: Tailoring Treatment Based on Mutations […]
Great blog about brain cancer provided me great information the genetic approach for treating it clearly is a good way to treat the cancer thanks for such a great blog.