From Rapid-Aging to Common Heart Disease
Last week the DNA Science blog looked at how Dr. Francis Collins became involved in the quest to discover the genetic defect that causes the rapid-aging disorder Hutchinson-Gilford progeria syndrome. Preliminary results of a possible drug therapy — one originally developed to treat childhood brain cancer — were about to be published. Dr. Collins isn’t on that paper, perhaps sidetracked with things like running the NIH.
We All Have Progerin
Between the progeria gene discovery in 2003 and the recent repurposed drug news lies perhaps the most important paper of all: a 2010 report comparing the arteries of two children with progeria who’d died of heart attacks – a girl just under age 10, and a boy aged 14 – to blood vessels from 29 people.
Not only did the researchers find progerin – the stunted protein at the root of progeria — in the arteries of normal individuals, but they calculated that build-up in nuclear membranes increases at the rate of 3.34% a year. A one-month old had progerin in one out of 1,000 blood vessel cells; a 97-year-old had 20 cells per thousand bogged down with progerin. The protein was even in people without cardiovascular disease risk factors (CVD).
Dr. Collins explained the significance of the finding. “We’ve learned that the same pathway that is activated strongly and prematurely in kids with progeria is also happening in you and me. That toxic protein they make from the beginning is also in our cells as we approach senescence. So cell senescence is not just a running down of the system – it’s an active process. A signal turns on this protein.”
And that aging signal, Dr. Collins added, is connected to the shortening of the chromosome tips that serves as a cellular clock. So the glimpse into aging the kids with progeria provide may have illuminated a new risk factor that can damage blood vessels even in a star athlete who eats only broccoli.
A Repurposed Drug
The beauty of identifying a gene that causes a disease when mutant is that it provides a target. Genetics offers precision.
Leslie Gordon, MD, PhD, who founded the Progeria Research Foundation (PRF) and is first author on the drug paper, remembers the gene discovery. “We didn’t have any idea that finding the gene mutation would do what it’s done for us, but we knew it was very, very important.” Soon after, the PRF broadened its mission: “To discover treatments and the cure for progeria and its aging-related disorders.”
Once researchers knew the enemy — progerin – the hunt for a treatment began. A class of drugs called farnesyl transferase inhibitors looked like they’d compensate for the basic defect in progeria, which is inability to remove a small organic molecule called farnesyl from one end of a protein called lamin A.
The progeria community lucked out, for lamin A was well-studied and already known to be behind several muscle and heart conditions. “The minute we found the gene mutation, we brought in great lamin biologists and learned all about what could be going wrong,” Dr. Gordon recalled.
Their luck continued when some of the biologists mentioned that Schering-Plough, now Merck, had been working for a decade on farnesyl transferase inhibitors and had already spent millions. Farnesyl enables the most common oncoprotein, ras, to alter cell signaling in a way that causes cancer. But ras finds its way around the drugs, and they failed in combating childhood brain cancer.
“Merck saw a chance to do something for children with progeria and they jumped at it. They supplied the drug at no cost. We approached Dr. Mark Kieran, who was conducting clinical trials with the drug on kids with brain cancer. He’d never seen progeria, so we invited him to a meeting, and he jumped right in,” said Dr. Gordon.
Dr. Kieran, a pediatric neurooncologist at Boston Children’s Hospital and the Dana-Farber Cancer Institute, picked up the story.
“When Francis Collins discovered the progeria mutation and found that the defect wasn’t an alteration in the encoded protein, but loss of a splice site that allows the molecule to remove its farnesyl site,” that suggested a drug mechanism. “Since there’s no way to fix the mutation, maybe we could prevent the abnormal molecule from adding on the farnesyl group, prevent the molecule from moving up in the nuclear membrane and getting stuck there and causing progeria. Would the farnesyl transferase inhibitors I was testing in kids with brain tumors prevent the toxic effects of progerin?”
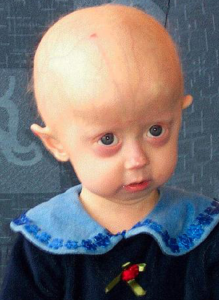
Clinical Trial Brings Elderly Kids From All Over
The PRF found 96 kids from 40 countries, and 25 of them have completed at least two years of the ongoing first clinical trial, testing the drug lonafarnib. “They were incredibly courageous. They trust us. They have to sign 40-page consent forms every 4 months. Some of these people have never been on a plane, and it takes them two days to get here. It was an unbelievable experience and privilege,” Dr. Gordon said.
“Many of the families had never seen snow. They have different cultures, languages, climate. It makes you aware of how different yet how similar we all are,” added Dr. Kieran.
So how do you test a drug to treat a disease that affects one in a million kids, striking them down with a heart attack or stroke? Using a placebo is unthinkable, and there are too few kids to worry about statistically significant sample sizes anyway. The researchers devised a clever approach: track weight.
“Each child served as his or her own control. These kids just stop growing at age 3. The curve is close to flat. So we predicted that if the drug really worked, we’d see an upturn in the rate of weight gain,” Dr. Collins explained.
And that’s what they saw: 9 of the 25 children had a greater than 50% increase in the rate of weight gain. In 6 kids rate of weight gain fell, possibly due to drug side effects of nausea and diarrhea. For ten kids, weight stabilized.
But what really excited the research team were the cardiovascular changes.
The researchers used a technique called pulsed wave velocity to assess stiffness of the arteries, because this is gentler than cardiac catheterization for these very fragile children. “Stiff arteries are a characteristic of the advanced atherosclerosis in these kids and older people with the same problem. Not only did we see the stiffening stop getting worse, but it got better!” said Dr. Collins.
Disease reversal appeared in echocardiography too. “The thickness of the carotid is considerably greater than it should be for a child this age, but the thickness reduced over the 2 years. That was unexpected and may be the most encouraging part of the trial,” Dr. Collins said. Some of the kids also developed stronger bones and better hearing.
In fact, each patient improved in some way. And while some people pointed out that the results were incremental, possibly even “noise,” I think that even small signs of improvement in an otherwise untreatable disease, based on countering the underlying disease mechanism, is a leap forward.
The researchers are careful to temper their excitement. “The only thing we can say now is we make parts of the cardiovascular system get better on the drug and assume cardiovascular risk will go down because of it. But what we think and what we believe are not the same as knowing. Time will tell,” said Dr. Kieran.
More kids have signed up for expanded clinical trials, which will test three additional drugs that have had promising results in mice, over at least the next dozen years.
Dr. Kieran put it all into perspective. “When we think that less than 10 years ago we didn’t know what this disease was … we didn’t know the gene, the protein, the molecule. And in 10 years we’re already going to a treatment that may change the course of the disease. The hope is that all four drugs will begin to make the rapid and significant changes needed to help kids in the long term.”
And when gene variants are discovered that track with progerin-mediated cardiovascular disease, a once-discarded drug candidate may find a huge new market, a possibility clearly not lost on the folks at Merck.
“Should we all start taking lonafarnib? No. But for researchers who want to understand healthy aging, and have a better chance to help those in whom aging is happening sooner than it should, we now have a conclusive study in which a rare disease turned out to provide good insight into a molecular mechanism,” said Dr. Collins.
The continuing story of conquering progeria is a glorious illustration of the value of basic research in discovering how something happens – and that’s the heart and soul of science.



Clinical Trial Brings Elderly Kids From All Over
Thank you, but i must say that there are a few things i dont agree with. Thanks
What don’t you agree with? Is there a factual error?